Frequently Asked Questions
The Ontario Court has authorized a Notice to inform Class Members about the approval of the Settlement Agreement in these Class Actions. The notice explains the lawsuits, the Settlement, and Class Members’ legal rights.
In a class action, one or more people called a “Representative Plaintiffs” sue on behalf of those with similar claims. These people are called a “Class” or “Class Members”. The courts resolve the issues for everyone affected by the class action, except those who excluded themselves, or “opt out” of the lawsuit.
The class actions relate to the M2a 38, M2a Magnum, or ReCap Femoral Resurfacing System hip implants, or any combination thereof, implanted in Canada and used as a metal-on-metal hip implant system (the "Biomet Devices"). The Plaintiffs claim that the Biomet Devices were defective and failed prematurely when implanted in patients in Canada. The Defendants deny these claims, and the Court has not decided whether the claims are correct.
The Plaintiffs and the Defendants have agreed to a Settlement of the class actions. By agreeing to settle the lawsuit, the parties avoid the costs, uncertainty, and delay of going to trial and obtaining judgment, and the risks associated with being unsuccessful at trial. In this case, it also means that Class Members will not need to testify in court.
The Ontario Superior Court of Justice approved the Settlement on October 28, 2024. A copy of Justice Glustein's decision to approve Settlement is available here.
The settlement applies to all eligible class members who were implanted with a Biomet Device in Canada and have not opted out of the Dine v. Biomet et al. action, their estates and certain family members.
Class Members must have been implanted with a Biomet Device in Canada to be eligible for compensation.
In order to participate, Class Members must submit a completed Claimant Declaration to the Claims Administrator by the applicable deadline.
Each Class Member must provide Product Identification confirming the reference number (sometimes referred to as “catalogue number”) and lot number of the device that was implanted, in addition to other documents required by the Settlement Agreement.
Product Identification confirms that Class Members were implanted with a Biomet Device. Product Identification can be found on the peel-and-stick label (the “Label”) from the Biomet Device that should be affixed to the medical record from the implant surgery (sometimes called the implant operative report).
Class Members can obtain a copy of their implant surgery medical record from the hospital where the implant surgery occurred or from a physician. For assistance, Class Members can contact one of the law firms for the Class Members ("Class Counsel") using the information online here: biometdevicesettlement.com/contact-us.aspx
To be eligible under the Settlement Agreement, the reference/catalogue number on the Label must be as follows:
- The claimant must submit a Product Identification for both a femoral head and a one-piece acetabular cup.
- The following reference/catalogue numbers correspond to femoral heads used with the M2a Magnum:
| 157442 | S031138 |
| 157444 | S031140 |
| 157446 | S061138 |
| 157448 | S061140 |
| 157450 | S121138 |
| 157452 | S121140 |
| 157454 | S331138 |
| 157456 | S331140 |
| 157458 | S661138 |
| 157460 | S661140 |
| S001138 | S991138 |
| S001140 | S991140 |
- following reference/catalogue numbers correspond to the acetabular cups used with the M2a Magnum:
US157844 US257844 US157846 US257846 US157848 US257848 US157850 US257850 US157852 US257852 US157854 US257854 US157856 US257856 US157858 US257858 US157860 US257860 US157862 US257862 US157864 US257864 US157866 US257866
The following reference/catalogue numbers correspond to the femoral heads or caps used with the M2a Recap:
| 157238 | 157256 | 157341 | US 157343 | 157145 | US 157140 |
| 157239 | 157257 | 157342 | US 157343 | 157146 | US 157141 |
| 157240 | 157258 | 157343 | US 157345 | 157147 | US 157142 |
| 157241 | 157259 | 157344 | US 157346 | 157148 | US 157143 |
| 157242 | 157260 | 157345 | US 157347 | 157149 | US 157144 |
| 157243 | US 157239 | 157346 | US 157348 | 154150 | US 157145 |
| 157244 | US 157241 | 157347 | US 157349 | 157151 | US 157146 |
| 157245 | US 157243 | 157348 | US 157350 | 157152 | US 157147 |
| 157246 | US 157245 | 157349 | US 157351 | 157153 | US 157148 |
| 157247 | US 157247 | 157350 | US 157352 | 157154 | US 157149 |
| 157248 | US 157249 | 157351 | US 157353 | 157155 | US 157150 |
| 157249 | US 157251 | 157352 | 157138 | 157156 | US 157151 |
| 157250 | US 157253 | 157353 | 157139 | 157157 | US 157153 |
| 157251 | US 157255 | US 157338 | 157140 | 157158 | US 157154 |
| 157252 | US 157257 | US 157339 | 157141 | 157159 | US 157155 |
| 157253 | 157338 | US 157340 | 157142 | 157160 | US 157156 |
| 157254 | 157339 | US 157341 | 157143 | US 157138 | US 157157 |
| 157255 | 157340 | US 157342 | 157144 | US 157139 |
- The following reference/catalogue numbers correspond to the acetabular cups used with the M2a Recap:
157844 157944 130846 130846 HA 1575438 157846 157946 130848 130848 HA 157440 157848 157948 130850 130850 HA 157442 157850 1557950 130852 130852 HA 157444 157852 157952 130854 130854 HA 157446 157854 157954 130856 130856 HA 157448 157856 157956 130858 130858 HA 157450 157858 157958 130860 130860 HA 157452 157860 157960 130862 130862 HA 157454 157862 157962 130864 130864 HA 157456 157864 157964 130866 130866 HA 157458 157866 157966 130868 130868 HA 157460 - The following reference/catalogue numbers correspond to the femoral heads used with the M2a 38:
11-173660 11-173661 11-173662 11-173663 11-173664 11-173665 11-173666 - The following reference/catalogue numbers correspond to acetabular cups used with the M2a 38:
15-105048 15-106048 RD118848 15-105050 15-106050 RD118850 15-105052 15-106052 RD118852 15-105054 15-106054 RD118854 15-105056 15-106056 RD118856 15-105058 15-106058 RD118858 15-105060 15-106060 RD118860 15-105062 15-106062 RD118862 15-105064 15-106064 RD118864 15-105066 15-106066 RD118868 15-105068 15-106068 RD118870 15-105070 15-106070
Where a Product Identification submitted by a claimant specifies a reference/catalogue number listed above, except that it includes or excludes an alphabetical prefix (e.g. "US"), the Claims Administrator shall deem the claimant to have submitted qualifying Product Identification for that component.
The images below are examples of Product Identifications. Please note that not all product labels are identical to the examples below.. These examples are provided to help Class Members identify the location of the reference and lot numbers of their device to assist them in determining whether they may be eligible under the Settlement.

If a Class Member is unable to obtain the Label because their implant surgery hospital could not locate it in their hospital medical records, then they may provide the following to prove that they received a Biomet Device:
a) If the Biomet Device has been explanted from the Class Member’s body and it still exists, they may provide (1) a colour photograph of the Biomet Device that shows the identification numbers on the edge of the Biomet Device, and (2) a Physician's Declaration confirming that they were implanted with a Biomet Device and the date of the implantation;
OR
b) If Class Members cannot obtain a photograph because the Biomet Device is not within their possession, custody, or control, they must provide (1) a copy of their implant surgery operative report from the hospital where they were implanted, which confirms that they were implanted with a Biomet Device, and (2) a Physician's Declaration confirming that they were implanted with a Biomet Device and the date of implantation.
Eligible Class Members who submit all required forms and documentation within the timelines in the Settlement Agreement will receive compensation.
The Settlement provides the following compensation amounts for Class Members who were implanted with Biomet Devices:
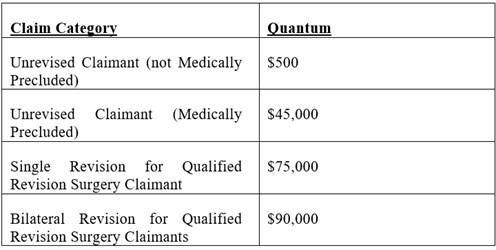
“Qualified Revision Surgery Claimant” means a Class Member who, as of the Claims Deadline, was implanted with a Biomet Device in Canada and:
i. has had a revision surgery;
ii. has been scheduled for a revision surgery; or
iii. was indicated by a physician as requiring a revision surgery and the revision surgery is planned, even if the date and time have not yet been finalized.
The revision must have taken place, or take place, at least 180 days after the Index Surgery and not have been required because of infection or trauma unless medical records establish that the claimant would likely have required the revision regardless of the infection or trauma.
“Medically Precluded” means a Class Member for whom a Revision Surgery was determined to be necessary within 12 years and 1 day of the Index Surgery, but who was unable to undergo a Revision Surgery due to the existence of a medical condition.
The Settlement Agreement provides that compensation for Qualified Revision Surgery Claimants and Medically Precluded Class will be subject to the following reductions:
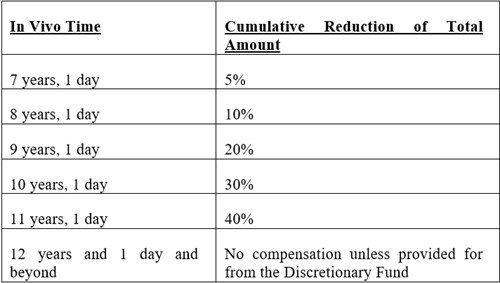
The Settlement Agreement also provides for:
a) A Discretionary Fund to be distributed to Class Members under the Special Claims Protocol;
b) Additional compensation for certain defined complications;
c) Compensation for certain out-of-pocket expenses; and
d) Compensation for family members who provided care in certain circumstances.
For class members resident outside of Quebec, a 10% levy on each award will be paid to the Ontario Class Proceedings Fund. For class members resident in Quebec, a 10% levy on each award will be paid to the Quebec Fonds d’aide aux actions collectives.
The Settlement Agreement states that Claimants may be eligible for up to an additional $40,000 in compensation for complications following a Revision Surgery. Claimants who had a Biomet Device implanted in both the left and right hips, and underwent Revision Surgeries in both hips, may be eligible for up to an additional $50,000.
The table below sets out the qualifying complications and available compensation:
| Complication | Single Claimant | Bilateral Claimant |
| Infection (any infection in the revised hip that is diagnosed within 30 days after a Revision Surgery and determined to have been caused by the Revision Surgery) | $10,000 | $12,500 |
| Permanent Nerve Damage (nerve damage [including but not limited to meralgia paresthetica and foot drop caused by peroneal nerve damage] resulting from a Revision Surgery that is permanent as established by medical records or a Physician’s Declaration, or that has persisted for 18 months or more. | $20,000 | $25,000 |
| Second Revision (surgery to remove a replacement hip implant that had been implanted as part of a Revision Surgery because the replacement hip device failed) | $20,000 | $25,000 |
| Blood Clot (diagnosis made within 72 hours of a Revision Surgery of pulmonary embolism or deep vein thrombosis that resulted from a Revision Surgery) | $10,000 | $12,500 |
| Stroke (cerebrovascular incident or insult occurring within 72 hours of a Revision Surgery and determined to have been caused by the Revision Surgery) | $40,000 | $50,000 |
| Third Revision (surgery to remove a replacement hip implant that had been implanted as part of a Second Revision because the replacement hip device failed) | $40,000 | $50,000 |
| Death (class member died within 72 hours after a Revision Surgery as a result of the Revision Surgery) | $40,000 | $50,000 |
| Femoral Fracture (fracture of femur that occurs during a Revision Surgery or as a result of the Revision Surgery, and does not include fracture that results from trauma that occurs before or after the Revision Surgery) | $16,000 | $19,000 |
| Dislocation (complete disassociation of femoral head and acetabular cup that occurs within 6 weeks of the Revision Surgery) | $12,000 | $15,000 |
| Lost Wages (economic loss supported by documentary evidence showing income loss in excess of 20% of the claimant’s aggregate gross income for the two highest earning years in the four years preceding the Revision Surgery) | $12,000 | $25,000 |
| Heart Attack (myocardial infarction or cardiac arrest occurring within 72 hours of a Revision Surgery and determined to have been caused by the Revision Surgery) | $40,000 | $50,000 |
| CAP | $40,000 | $50,000 |
For assistance with a claim for compensation arising from a qualifying complication, Class Members can contact one of the Class Counsel law firms using the information online here:biometdevicesettlement.com/contact-us.aspx
Under the Settlement Agreement, the Defendants agreed to pay an additional $750,000 for distribution through the Special Claims Protocol. The Court approved the Special Claims Protocol by order dated October 28, 2024.
The Special Claims Protocol provides compensation for claims which are not covered under the main Settlement Agreement. In particular, the Special Claims Protocol provides compensation for:
(a) Unrevised Class Members with high blood levels of cobalt or chromium;
(b) Any Class Members whose index surgery occurred in late 2013 or 2014 and whose revision surgery occurred within 12 years but after the claims deadline in the Settlement; and
(c) Class Members whose revision surgeries occurred 12-16 years after their index surgery.
For assistance with a claim under the Special Claims Protocol, Class Members can contact one of the Class Counsel law firms using the information online here: biometdevicesettlement.com/contact-us.aspx
Under the Settlement Agreement, the Defendants agreed to pay Class Counsel $1.25 million as a contribution towards Class Counsel Fees, Disbursements and applicable taxes. The Court approved the Defendant's contribution toward Class Counsel Fees, Disbursements and applicable taxes.
The Court also approved Class Counsel Fees and Disbursements of 25% to be deducted from payments made to eligible Class Members for the work performed in the class action and to obtain the Settlement.
Further legal fees, disbursements, and taxes may be payable if a Class Member agrees with their lawyer that those amounts will be paid. Class Counsel has undertaken not to charge more than 8.3% to assist with the Class Member’s claim.
Class Members may choose to retain a different lawyer (other than Class Counsel) to assist with a claim or may choose to submit a claim without assistance from a lawyer.
The Claims Administrator for this Class Action is Verita Global LLC. The Claims Administrator can be contacted at: 1-833-419-4973 or [email protected].
To receive benefits under this Settlement Agreement, Class Members must submit a completed Claimant Declaration along with a Physician’s Declaration (if applicable) before the applicable deadlines. These forms may be submitted online here and are available as printable PDFs here.
For Class Members who are unrevised, medically precluded from having a revision surgery, or have had a revision surgery as of October 27, 2025, all required documents supportting their claim must be submitted by January 26, 2026.
For Class Members who have not yet had a revision surgery but, as of the Claims Deadline, have a scheduled revision surgery or have been indicated by a physician as requiring a revision surgery that has been planned (even if the date and time have not yet been finalized), a claim must be submitted by January 26, 2026. All further required documents in support of their claim must be submitted within 90 days of the date on which the scheduled revision surgery takes place.
For Class Members who have undergone a revision surgery between October 27, 2025 and January 26, 2026, all required documents in support of their claim must be submitted within 90 days after the revision surgery.
A “Scheduled Revision Surgery” means that the claimant has been scheduled to receive a Revision Surgery or a Revision Surgery has been planned (even if the date and time have not yet been finalized), but the Revision Surgery has not occurred as of 270 days after the date on which the Notice of Settlement Approval was disseminated, evidenced by the claimant submitting to the Claims Administrator by the Claims Deadline documentation in the form of:
a) Documentation from a hospital or physician confirming the claimant has been scheduled to receive a Revision Surgery, but the Revision Surgery has not occurred as of 270 days after the date on which the Notice of Settlement Approval was disseminated; or
b) A completed Physician’s Declaration, which confirms that: (i) the Revision Surgery has been scheduled as of the Claims Deadline; or (ii) the claimant has been indicated by a physician as requiring a Revision Surgery as of the Claims Deadline and the Revision Surgery has been planned (even if the date and time have not yet been finalized), in either case including the date on which the need for a Revision Surgery was indicated.
If a Class Member's Revision Surgery cannot occur due to a documented medical condition, the Class Member may be eligible to receive compensation under the Settlement Agreement as a Medically Precluded Class Member. In that case, the Class Member must submit a Claimant Declaration along with appropriate documentation that reflects this status (as provided in the Settlement Agreement) on or before January 26, 2026.
Class Counsel are the law firms Koskie Minsky LLP, Whelton Hiutin LLP, Klein Lawyers LLP, and Sylvestre Painchaud et Associés.
The Class Counsel law firms can be contacted through the information here: biometdevicesettlement.com/contact-us.aspx
